
Self-assembly at surfaces
Molecular self-assembly of surface-confined architectures is a prospective candidate for fabricating functional nanostructures with atomic precision on large areas. Employing reversible interacons enables the growth of nanostructures in a dynamic equilibrium with their surroundings. Reversible interacons enable the correction of transient defecve motifs, resulting in highly ordered structures. Self-selection and self-recognition enable the selection of a particular component from multicomponent mixtures and its attachment to the desired position; they are possible thanks to the dynamic error correction process.
For efficient error correction, the bonding should be reversible at temperatures at which the employed molecules remain stable. This precludes strong covalent bonds; therefore, weaker intermolecular interactions should be used instead. These include hydrogen and halogen bonds between suitable functional groups and coordination bonds between metal atoms and ligands. Another condition of efficient error correction is free diffusion of the molecules over the substrate, which ensures that "correct" binding partners reach a particular site.
Both conditions require the system to be close to thermodynamic equilibrium to suppress kinetic limitations. In the thermodynamic equilibrium, the islands of the condensed molecular phase are in a dynamic equilibrium with their surroundings, i.e., free molecules that diffuse on the bare substrate. These molecules attach to existing islands at the same rate as other molecules detach. The self-assembled phases are, therefore, not stable per se, but the surrounding environment should always be considered.
One of the kinetic strategies to form long-range ordered organic or metal-organic networks is to prevent the formation of defective motifs. We have employed this strategy to build the metal-TCNQ networks on graphene by controlled co-deposition of both components. Another one is the controlled kinetics of one of the processes in the self-assembly (deprotonation reaction) using an electron beam.
Concerning the kinetics, we have revealed that transformations of self-assembled phases exhibits a wide variety of phenomena. Some of them are common also for inorganic phases like Ostwald ripening. The other, the burst nucleation of the new phase, is a characteristic of the molecular islands at surfaces close to the thermodynamic equilibrium. Later, we extended the burst nucleation concept to burst transformation.
Metal-Organic Frameworks on Graphene
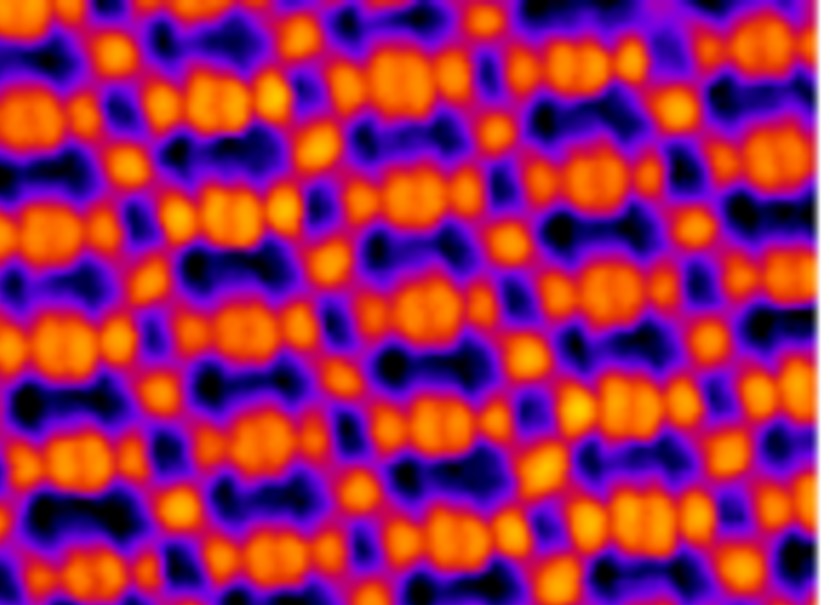
We have successfully synthesized metal-organic frameworks (MOFs) M-TCNQ (M = Ni, Fe, Mn) on epitaxial graphene/Ir(111), showing a single phase with M 1(TCNQ)1 stoichiometry. We demonstrate a remarkable chemical and thermal stability of TCNQ-based 2D MOFs: all the studied systems survive exposure to ambient conditions, with Ni-TCNQ doing so without any significant changes to its atomic-scale structure or chemical state. Thermally, the most stable system is Fe-TCNQ which remains stable above 500 °C, while all the tested MOFs survive heating to 250 °C. Overall, our system combines the atomic-scale definition required for fundamental studies with the robustness and stability needed for applications; thus, we consider it an ideal model for research in single-atom catalysis, spintronics, or high-density storage media.
Z. Jakub et al.: Remarkably stable metal-organic frameworks on an inert substrate: M-TCNQ on graphene (M = Mn, Fe, Ni). Nanoscale 14 (2022), 9507.
Tunable Dipolar Layers from Carboxylic Acids
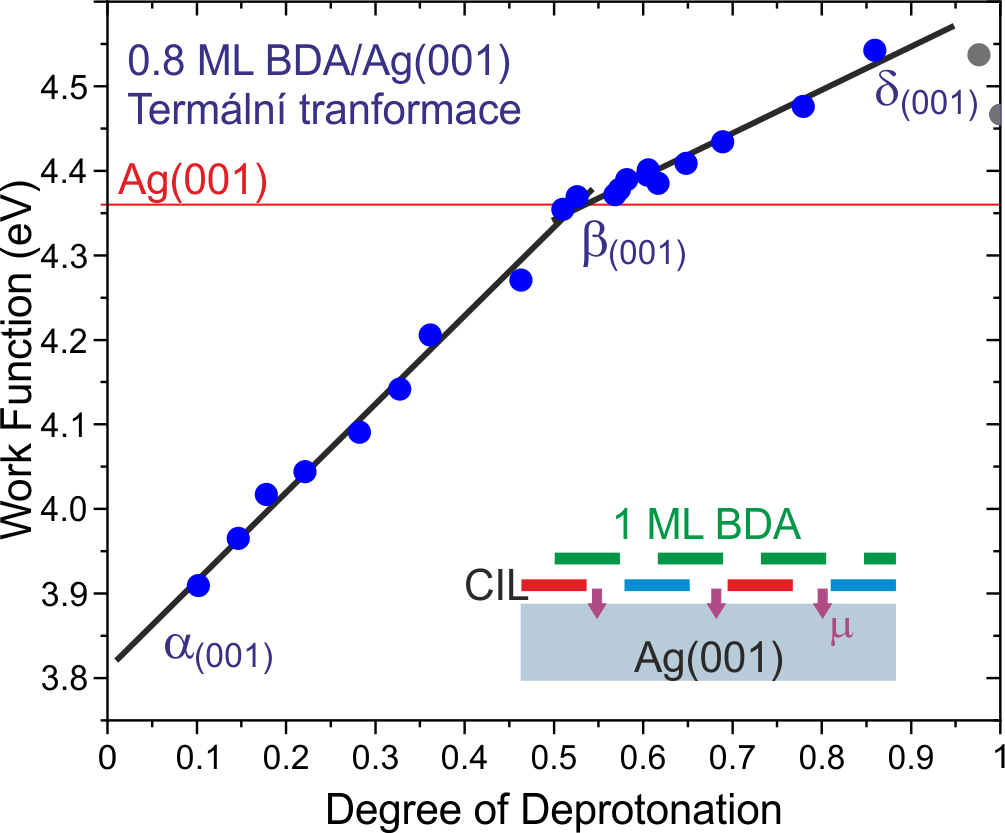
We have demonstrate monolayer thick charge injection layers (CILs) based on aromatic carboxylic acids that can induce an energy level shift in the subsequent layers by up to 0.8 eV. By gradually transforming the as-deposited molecules, we achieve a highly tunable energy level shift in the range of 0.5 eV. We reveal that the work function and energy-level positions in the CIL increase linearly with the density of induced dipoles. The energy level position of the subsequent layers follows the changes in the CIL. Our results thus connect the energy alignment quantities, and the high tunability would allow precise tuning of the active layers deposited on the CIL, which marks a path towards efficient charge injection layers on metal electrodes, which are required to reduce contact resistance and enhance the efficiency of organic-semiconductor-based devices.
V. Stará et al.: Tunable Energy Level Alignment in the Multilayers of Carboxylic Acids on Silver. Phys. Rev. Appl. 18 (2022), 044048.
Phase Transformations
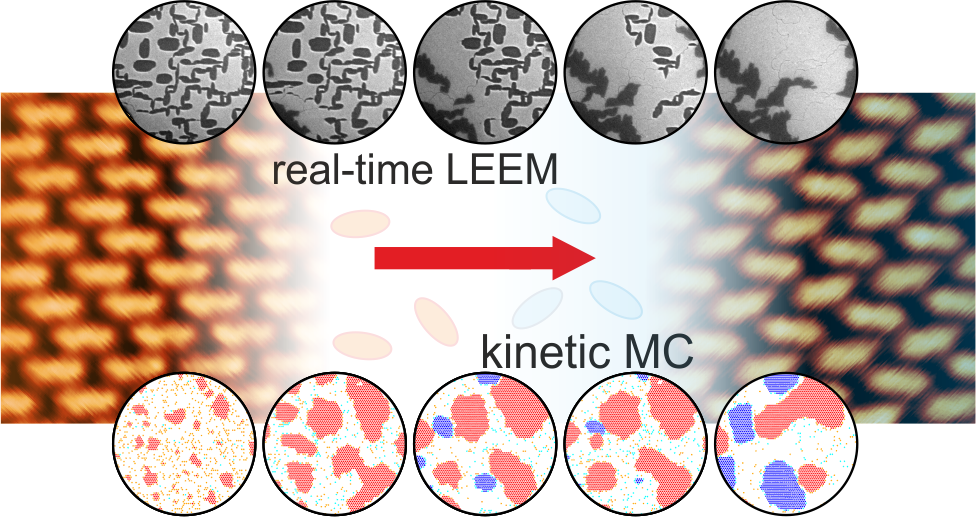
Low-energy electron microscopy (LEEM) is a surface-science method that enables mesoscopic surface imaging in real-time. We have employed real-time LEEM to visualize a phase transformation induced by the carboxylation of 4,4’ biphenyl dicarboxylic acid (BDA) on Ag(001) under ultra-high vacuum conditions. In the combination with kinetic Monte Carlo simulations, we reveal that the phase transformation exhibits a rich variety of phenomena. Some of them are common also for inorganic phases like Ostwald ripening. The other, the burst nucleation of the new phase is a characteristic of the molecular islands at surfaces that are close to the thermodynamic equilibrium.
P. Procházka, et al.: Multiscale Analysis of Phase Transformations in Self-Assembled Layers of 4,4’-Biphenyl Dicarboxylic Acid on the Ag(001) Surface, ACS Nano 14 (2020), 7269.
Full Layer Transformation
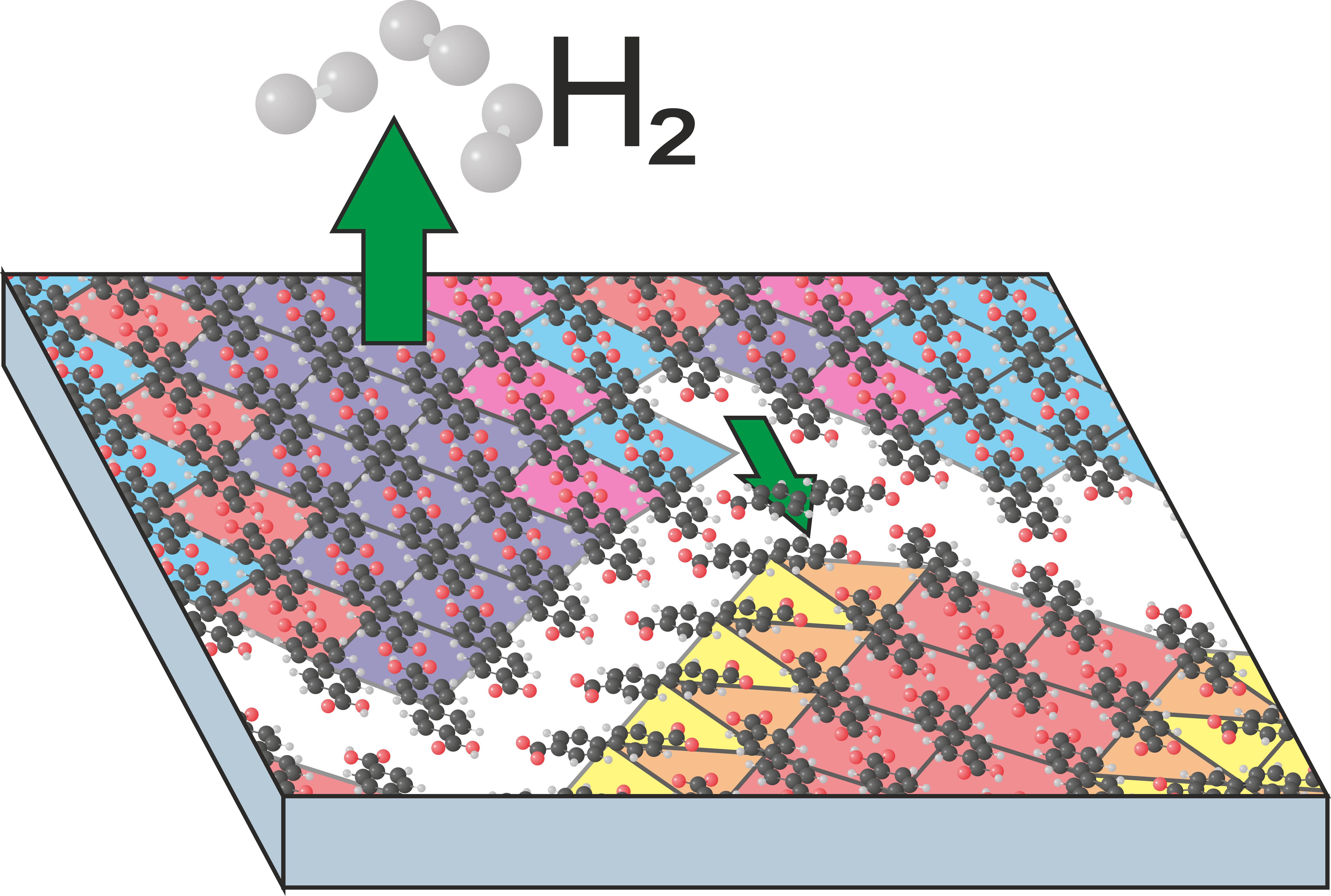
BDA molecules comprise two carboxyl groups, which can be thermally deprotonated at Ag(001) surface. As these groups are negatively charged the molecular structure should be changed and molecular phases irreversibly transform into different ones. In the full molecular layer covering the entire surface, the phase transformation proceeds differently from sub-monolayer coverages. There no space to grow phases with a lower density and molecules cannot diffuse or reorient. As a result new, compressed, phases are formed if possible. If not, the excessive molecules are expelled making space for the standard sub-monolayer phases.
P. Procházka, L. Kormoš, A. Shahsavar, V. Stará, A. O. Makoveev, T. Skála, M. Blatnik, J. Čechal: Phase Transformations in a Complete Monolayer of 4,4’-Biphenyl-Dicarboxylic Acid on Ag(001). Appl. Surf. Sci. 547, (2021), 149115.
k-Uniform Tiling
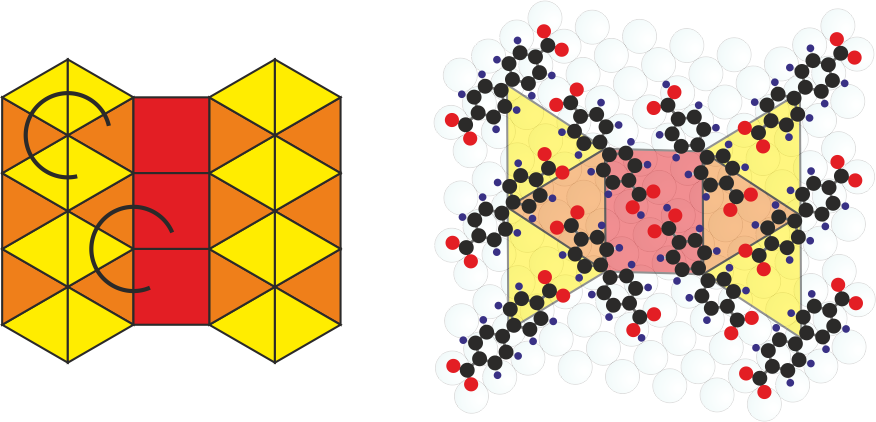
Tilings are the mathematical concept that enables to describe tesselation of the plane into polygons. We have shown that k-uniform tilings comprising several distinct tiles can be prepared from differently deprotonated biphenyl dicarboxylic acid (BDA) molecules. These are obtained by their controlled chemical transformation on the Ag(001) surface. The partially deprotonated BDA mediates the seamless connection of two distinct binding motifs in a single long-range ordered molecular phase and thus enables the connection of distinct tiles together resulting in the realization of 2- and 3-uniform tilings.
L. Kormoš, P. Procházka, A.O. Makoveev, J. Čechal, Nat. Commun. 11 (2020), 1856.
Step Edge Passivation
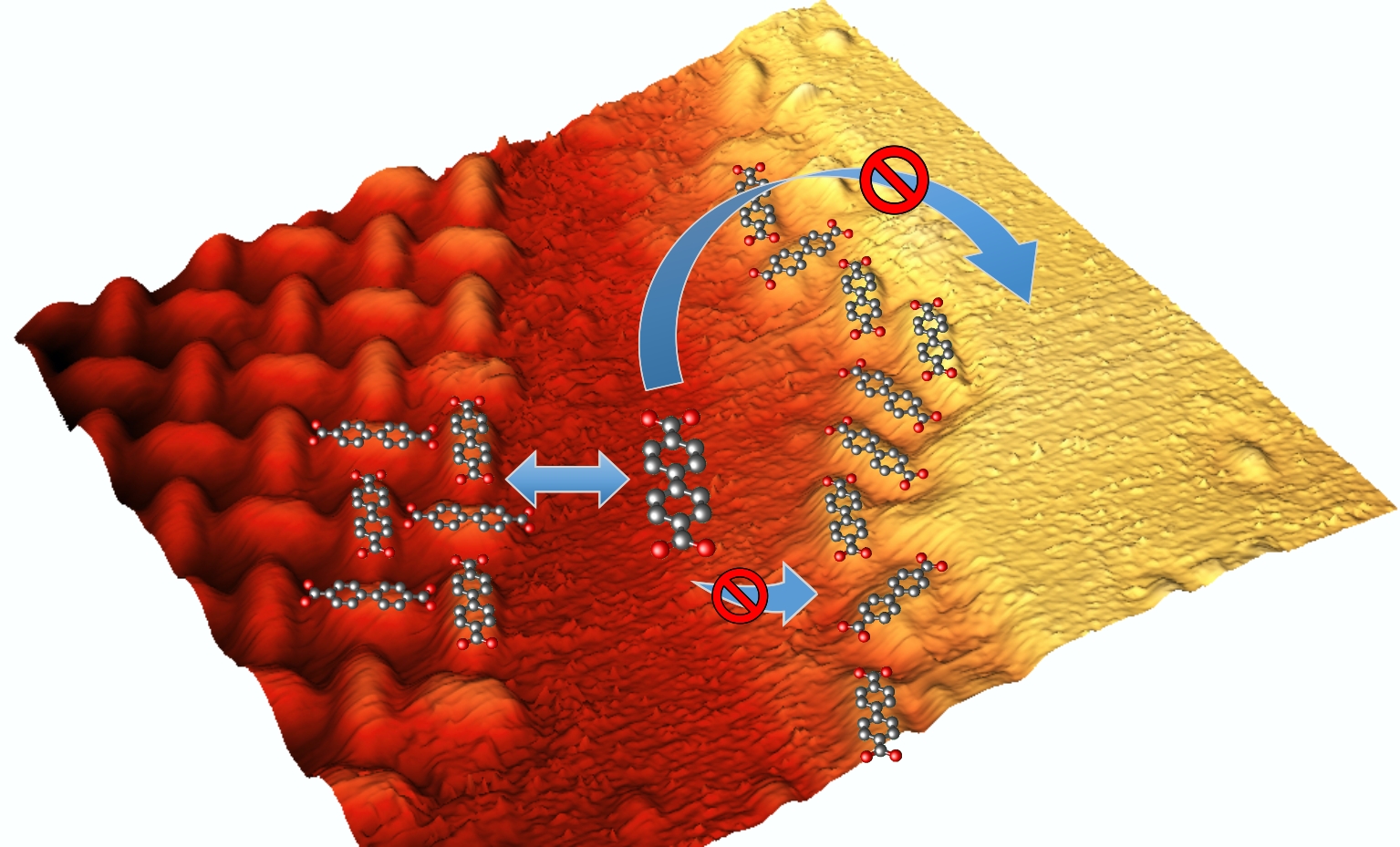
Our model molecule, BDA, is almost instantly deprotonated at the Cu (001) surface. The deprotonated groups are strongly interacting with the substrate and even more with the step-edge atoms; this results in step-edge decoration by BDAs. As the other side of BDA is also deprotonated, the densely arranged carboxylates will prevent attachment of any further BDA's causing an effective step edge passivation. This limits the BDA diffusion over the step edges and the attachment of additional BDA molecules preventing nucleation and growth of molecular islands on the step edges.
L. Kormoš, P. Procházka, T. Šikola, J. Čechal, J. Phys. Chem. C 122 (2018), 2815.

